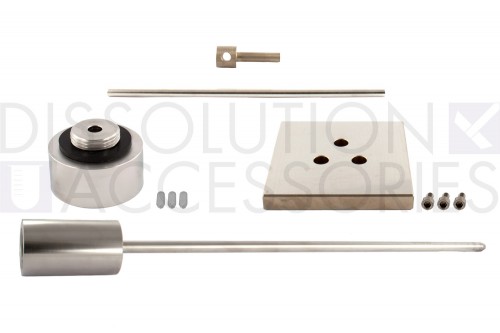
Intrinsic dissolution apparatus
PSIDASET-01
Intrinsic dissolution apparatus
The Intrinsic Dissolution Apparatus is an updated version of the original Wood Apparatus for intrinsic dissolution. The design includes a hardened punch, a controlled surface area and an improved die design for more reproducible results. It is designed to meet or exceed current USP specifications as described in USP Physical Test <1087>. The ground and hardened surface plate provides an ideal surface for compressing samples. The intrinsic dissolution apparatus is designed for use with standard USP Apparatus 1 and 2 dissolution testers.
Includes:
Intrinsic dissolution apparatus
| Product number | PSIDASET-01 |
| OEM | Agilent |
The Intrinsic Dissolution Apparatus is an updated version of the original Wood Apparatus for intrinsic dissolution. The design includes a hardened punch, a controlled surface area and an improved die design for more reproducible results. It is designed to meet or exceed current USP specifications as described in USP Physical Test <1087>. The ground and hardened surface plate provides an ideal surface for compressing samples. The intrinsic dissolution apparatus is designed for use with standard USP Apparatus 1 and 2 dissolution testers.
Includes:
The intrinsic dissolution rate is defined as the rate of dissolution of a pure pharmaceutical active ingredient when conditions such as surface area, agitation-stirring speed, pH and ionic-strength of the dissolution medium are kept constant. The determination of this parameter allows for screening of drug candidates and an understanding of their solution behaviour under different bio-physical conditions.
An Intrinsic Dissolution Testing Apparatus is a test system adapted for this dissolution testing of active Pharmaceutical Ingredients (APIs). The intrinsic dissolution rate of APIs are measured in order to depict the dissolution rate of a drug and determine its batch to batch chemical equivalency.
Dissolution rates often vary considerably with solid form. Dissolution tests are often used to ensure that production processes are under control.
The aqueous solubility of drug substances plays an important role in the formulation of drug dosage forms. For the oral route of administration it is well experienced that, unless the substance has an aqueous solubility above 10 mg/ml over the pH-range 1-7, then potential absorption problems may occur. A solubility less than 1 mg/ml is likely to give dissolution-rate limited absorption because solubility and dissolution rate are interrelated.
A solid substance can exist in several crystals forms. Suppose, for example, that one such a solid state (crystal) form is much less soluble than another. Perhaps one differs in its morphology or other physical properties. Either case may increase the difficulty of handling it in the manufacturing process, regardless of the dosage form
For solid oral dosage forms, the solubility of the active ingredient is a significant factor in our level of regulatory concern. Qualitative solubility studies have likely been completed at a much earlier phase of development. Quantitative solubility information, especially as a function of pH, may determine the extent of our regulatory concern about the different solid forms.
For less soluble drug substances, a determination of the intrinsic dissolution rate may support the specifications and methods for measuring dissolution.
Let's talk about that property for a moment. For example, if a solid drug has a specification for dissolution, it will be expressed as a figure such as not less than 80% in 15 minutes in a medium such as 0.1M hydrochloric acid, which approximates the conditions found in the stomach. To the drug developer, rapid dissolution in the stomach is often critical, so there may be good reasons to prefer to manufacture tablets or capsules from crystalline forms which are less stable, and thus more soluble. Unfortunately, this may increase the possibility of undesirable phase changes.
An Intrinsic Dissolution Testing Apparatus is a test system adapted for this dissolution testing of active Pharmaceutical Ingredients (APIs). The intrinsic dissolution rate of APIs are measured in order to depict the dissolution rate of a drug and determine its batch to batch chemical equivalency.
Dissolution rates often vary considerably with solid form. Dissolution tests are often used to ensure that production processes are under control.
The aqueous solubility of drug substances plays an important role in the formulation of drug dosage forms. For the oral route of administration it is well experienced that, unless the substance has an aqueous solubility above 10 mg/ml over the pH-range 1-7, then potential absorption problems may occur. A solubility less than 1 mg/ml is likely to give dissolution-rate limited absorption because solubility and dissolution rate are interrelated.
A solid substance can exist in several crystals forms. Suppose, for example, that one such a solid state (crystal) form is much less soluble than another. Perhaps one differs in its morphology or other physical properties. Either case may increase the difficulty of handling it in the manufacturing process, regardless of the dosage form
For solid oral dosage forms, the solubility of the active ingredient is a significant factor in our level of regulatory concern. Qualitative solubility studies have likely been completed at a much earlier phase of development. Quantitative solubility information, especially as a function of pH, may determine the extent of our regulatory concern about the different solid forms.
For less soluble drug substances, a determination of the intrinsic dissolution rate may support the specifications and methods for measuring dissolution.
Let's talk about that property for a moment. For example, if a solid drug has a specification for dissolution, it will be expressed as a figure such as not less than 80% in 15 minutes in a medium such as 0.1M hydrochloric acid, which approximates the conditions found in the stomach. To the drug developer, rapid dissolution in the stomach is often critical, so there may be good reasons to prefer to manufacture tablets or capsules from crystalline forms which are less stable, and thus more soluble. Unfortunately, this may increase the possibility of undesirable phase changes.